In early September 2018, the Ministry of Health and Welfare of Taiwan issued the revised provisions of the “Administrative Measures for the Implementation or Use of Medical Devices for Specific Medical Technology Inspection and Inspection”. The application of open cell therapy technology includes autoimmune cell therapy and cancer patients and entities for standard treatment failure. Patients with advanced cancer. The Shifu Cell Program will rapidly promote ADCTA to more drug-free or terminally fatal cancers, such as metastatic lung cancer, colorectal cancer, pancreatic cancer, prostate cancer, recurrent ovarian cancer, and breast cancer. Wait. Shifu Cell has cooperated with many hospitals in North, Central and South Taiwan, and has been able to formally serve Taiwan's medical technology and cancer patients in the near future. It has always adhered to the world's pioneering and therapeutic leader of Taiwan's immune cell therapy, and hopes to become a global standard for immune cell therapy through more successful cases of treating clinical patients.
TFDA Specific Law
Cellular treatment technology
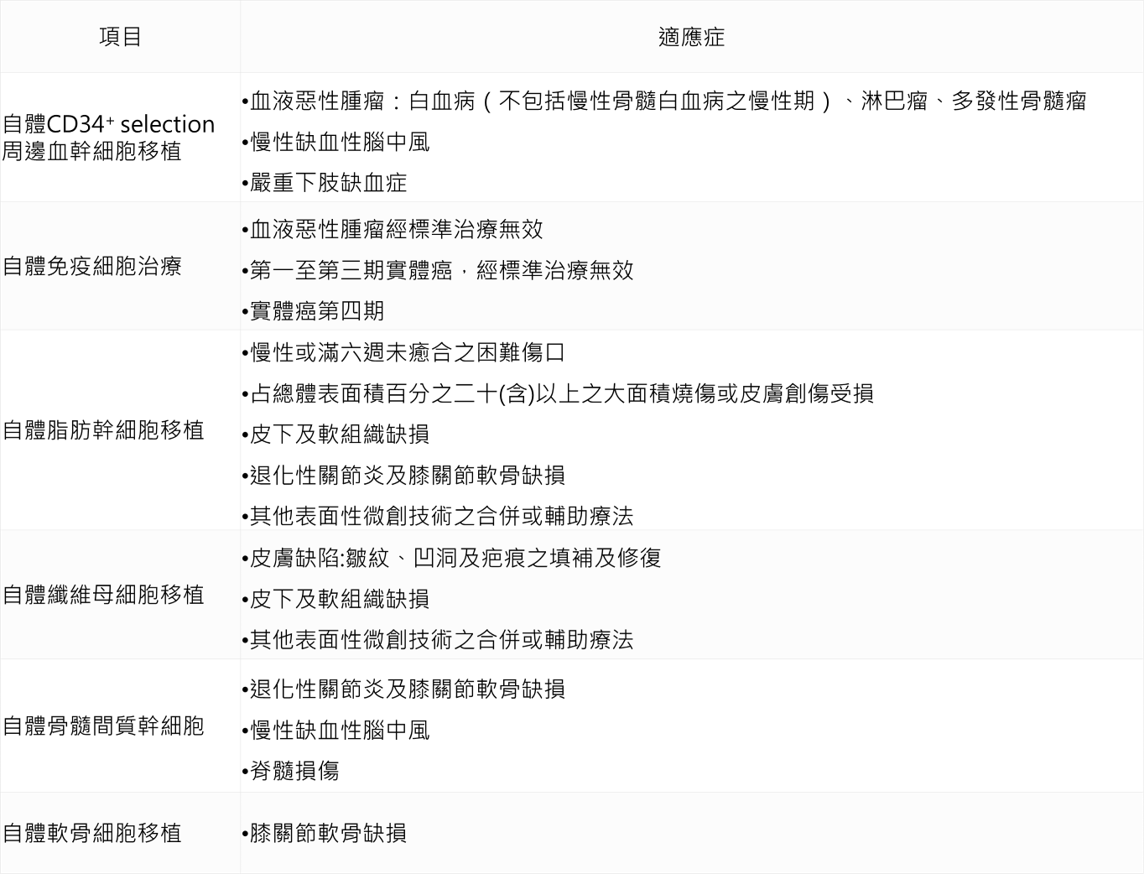
On September 4, 107, Weifu Department issued the “Administrative Measures for the Implementation or Use of Medical Devices for Specific Medical Technology Inspection and Inspection” (referred to as the special management method). On September 6, the six cell therapy technologies were officially opened, including the self-contained CD34+ selection. Blood stem cell transplantation, autoimmune cell therapy, autologous adipose stem cell transplantation, autologous fibroblast transplantation, autologous bone marrow mesenchymal stem cell transplantation, autologous chondrocyte transplantation.