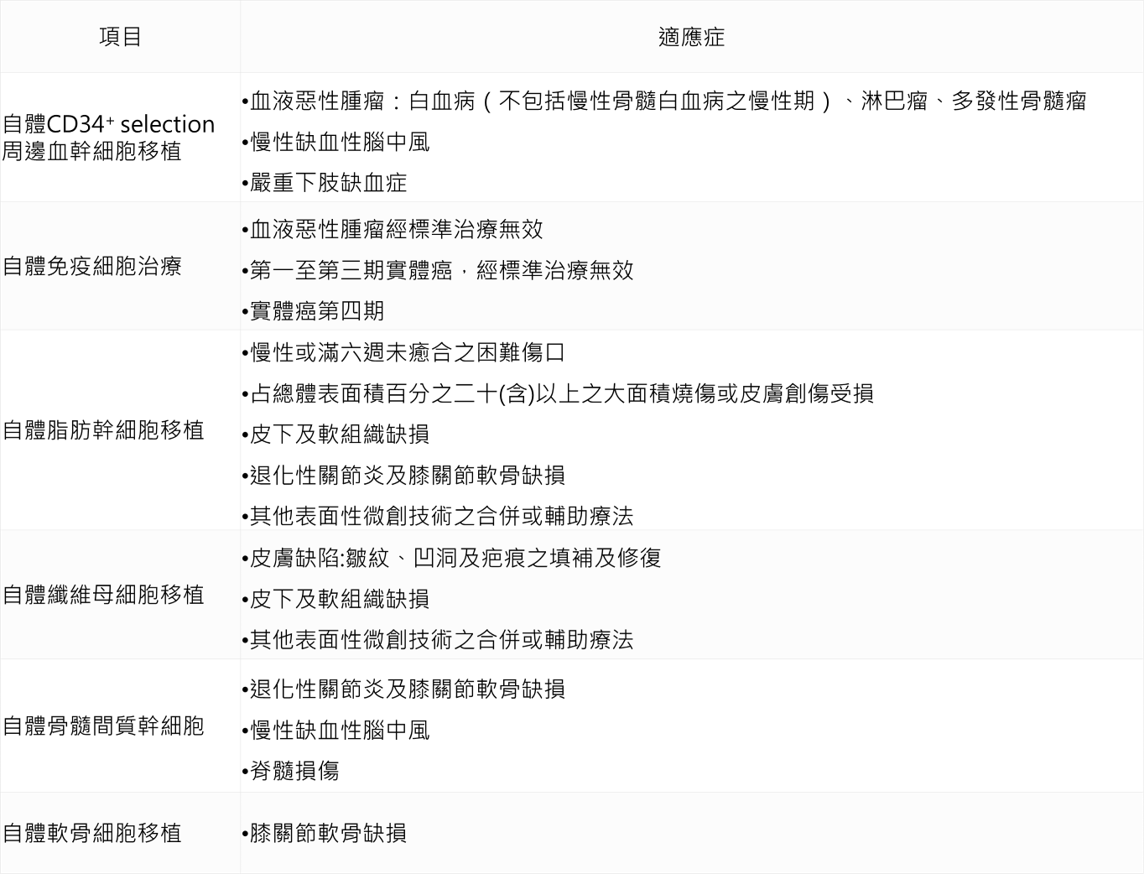
Cellular treatment technology
On September 4, 107, Weifu Department issued the “Administrative Measures for the Implementation or Use of Medical Devices for Specific Medical Technology Inspection and Inspection” (referred to as the special management method). On September 6, the six cell therapy technologies were officially opened, including the self-contained CD34+ selection. Blood stem cell transplantation, autoimmune cell therapy, autologous adipose stem cell transplantation, autologous fibroblast transplantation, autologous bone marrow mesenchymal stem cell transplantation, autologous chondrocyte transplantation.